What Are Polymers ? What is Addition polymerisation?
Polymers
Introduction
Polymers are
macromolecules (Large molecule) made from simple molecules and characterized by
repeating units in linear sequence. They are held together by covalent bonds. The simple molecules could be same or different.
The word
polymer was derived by Greek word Polymeros where poly means many and meros means parts. This word was given by Berzelius in 1853.
The simple
molecules which combines to form a polymer of high molecular weight are called
monomers. They are basic units of polymers.
Polymerisation
is a process in which large numbers of monomers combine to form a macromolecule
called polymer molecule.
The average
number of monomer molecules present in the polymer molecule is called degree of
polymerization (n).
Polymers are studied in the fields of polymer science, biophysics, and material science and engineering.
Classification of polymers
They are classified in many
ways by their origin, by the type of monomers used, by the properties of
polymers.
nomers used, by the properties of
polymers.
- Natural polymers are the substances that exists in nature. They can be obtained from plants, animals, and microorganisms, e.g., cellulose, starch, natural rubber, protiens, wool, silk, etc.
- Synthetic polymers are the substances that are prepared in laboratory or industry. They are also known as man-made polymers. e.g., nylons, terylene, plastics, polystyrene, etc.
- The polymers prepared from the polymerisation of same type of monomers in the polymer chain are Homopolymers.
- The polymers prepared from the polymerisation of different type of monomers in the polymer chain are Copolymers.
- Other polymers are classified on their physical properties.
- Plastics
- Fibres
- Resins
- Elastomers
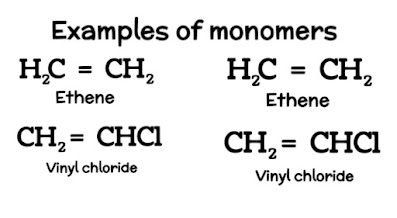
Addition Polymers
The unsaturated monomers are
added to each other to form the addition polymers.
In
polymerisation of addition polymers it involves in numbers of steps and hence
it is also called chain growth polymerization. The reaction is carried out by
formation of intermediate radical, cation, or anion.
This polymerisation involves three steps
in chain reaction that are initiation, propagation, and termination reaction.
- Polyethene (PE) : It is the most common plastic used in today life. It is a addition polymer. It is the mixture of similar polymers of Ethylene. It is a type of thermoplastic as it is harder at room temperature and get softer when heated.
There are two types of polyethenes.
1) Low density polyethene (LDPE) is prepared by free radical mechanism at high pressure.
Properties - It is partially crystalline solid. It is chemically inert, tough and flexible.
Uses - LDPE films are of high strength they are widely used for packaging, wrapping good products, textile, etc.
In agriculture, the flims are used to line the canals and ponds for preventing seepage of water
2) High density polyethene (HDPE) is prepared by co-ordination polymerisation in presence of Ziegler-Natta catalyst.
Properties - It is highly crystalline solid. It has greater hardness and tensile strength.
Uses - For making bottles, containers, buckets, crates and other housewares.
Used in cable insulation.
- Polypropylene (PP) : It is a thermoplastic used in large variety of applications. It is produced in chain-growth polymerisation.
Preparation : Propene if first dissolved in n-hexane and polymerisation is carried out at 20-100°C under a pressure of 1 to 40 atm, in the presence of catalyst TiCl3 .
Properties : The polymerisation by catalyst gives stereoregular polymer. It is highly crystalline isotactic polymer.
Uses : It is used in making pipes, strong tanks and seat covers.
Polypropylene fibers are used for making synthetic carpets.
- Polyvinyl chloride (PVC) : Polyvinyl chloride is the world's third-most widely produced synthetic plastic polymer. It is high strength thermoplastic materials widely used in application. It is a white, brittle solid material available in powder form or granules. It is widely used in buildings and constructions.
Preparation : It is prepared by free radical polymerisation in an inert solvent in presence of a peroxide.
Properties : It is a high strength thermoplastic polymer. It is flexible, having low crystallinity and dissolves in a mixture of acetone and CS2.
Uses : In manufacture of raincoats, hospital sheets, floor covers, etc.
As cable insulators, footware, etc.
- Polytetrafluoroethylene (Teflon or PTFE) : Tetrafluoroethylene is a fluorocarbon with the chemical formula C₂F₄. It is the simplest perfluorinated alkene.
Preparation : The emulsion of tetrafluoroethelene is subjected to free radical polymerisation using a peroxide. As the reaction is highly exothermic, efficient stirring and heat transfer is required.
Properties : It is tough material known as engineering plastic. It is resistant to heat, chemicals and a bad conductor of heat and electricity.
Uses : Used for coating articles and cookware for making nonstick utensils.
For preparing baskets, pump packing, valves, seals and non-lubricated bearings.
- Polystyrene (PS) : Polystyrene is a plastic used in a variety of situations, including construction, laboratory equipment and food packaging.
Preparation : Styrene monomer is prepared first from ethene and benzene by Friedel Crafts reaction followed by dehydrogenation with ZnO at 600°C.
Styrene is then polymerised under free radical conditions using benzoyl peroxide as initiator. The free radical polymerisation results in atactic polymer which is amorphous. But catalytic polymerisation gives a crystalline polymer.
Properties : It is a linear polymer, which is chemically resistant to acid and alkalies. It dissolves in organic solvents.
Uses :- The copolymer of styrene can be sulphonated and used as ion-exchange resins.
When air is passed into the molten polymer, styrofoam is obtained which is used for making cups, plates, packing consumers items.










Comments
Post a Comment